Chemical Equilibrium Is the Result of Chegg
Olmstead and Williams Chemistry Chapter 14 all sections Purpose. This can be easily said as the concentrations of the products increase the rate of.

Solved 1 Chemical Equilibrium Is The Result Of A All Of Chegg Com
Le Châ teliers Principle NAME.

. The concentration of the reactants and products change continually c. Chemical equilibrium can be understood as when the forward reaction is equal to the reverse reaction. The first test tube is used to compare color with what will be happening in the other test tubes.
Chemical Equilibrium Lab Report. We are going to use our knowledge of the Le Chateliers principle in. Introduction Chemical Equilibrium No chemical reaction goes to completion.
CHEM 1002 Laboratory 3. This experiment is based on Experiment 19A in Heath Laboratory Experiments. O opposing reactions attaining equal rates.
The system is said to be in chemical equilibrium. Rate fwd Rate reve. Introduction In a chemical reaction chemical equilibrium is a dynamic process where the systems rate of forward reaction equals that of the backward reaction.
In 1888 Henri-Lewis Le Châtelier described this phenomenon in a principle that states When a change in temperature pressure or concentration disturbs a system in chemical equilibrium the change. Chemical equilibrium results if FORWARD REACTION RATE EQUALS REVERSE REACTION RATE. This is referred to as the Law of mass action.
B stoppage of further reaction. Stoppage of further reaction. The position of the equilibrium describes the relative amounts of reactants and products that remain at the end of a chemical.
Chemical equilibrium is the result of all of the reactants being converted into products. IntroductionGoals of Experiment Past experiments have dealt with the effect of concentration and temperature on the initial rate of the reaction. A chemical reaction reaches equilibrium when the concentrations of the reactants and products no longer change over time.
Application of the law of chemical equilibrium to solubility equilibrio Solutions mixed Relative amounts of precipitate if any 1 CaCllaq HC0aq 2 CaCl aq KC0 aq 3 Results of 1 6 M HCI 4 Results of 3 6 MNH 5 CaClaq 6 MNH Noras noven as test tue 2 less chudly March tobeles Classy Some coudness attle Cloudy None. Both the forward and reverse reactions have stopped d. 1When a chemical system is at equilibriuma.
When equilibrium is reached both reactants and products are present at the same time. K w H OH- 1x10-7 1x10-7 1x10-14 Part 1 Chemical Equilibriumm Day 1 This experiment involves the qualitative description of some of the equilibrium systems before and after the stress is placed on the system. Chemistry questions and answers.
If we keep the temperature constant then these equilibrium concentrations will remain constant over time. Chemical Equilibrium Lab Report. Which of the following processes is.
In this experiment we will study the equilibrium properties of the reaction. This phase is typically reached when the rate of forward reaction is equal to the rate of. 1 Chemical equilibrium is the result of A all of the reactants being converted into products.
The goal of the experiment was to visualize this equilibrium by observing color change and determining what color changes mean in terms of. When a reaction stops some amount of reactants remain. To recognize the macroscopic properties of four chemical systems at equilibrium.
Chemical Equilibrium 1 Reading. Step 1 5 mL of the red complex ion solution FeNCS2 was poured into 4 test tubes. H 2 O H OH-And the equilibrium expression for the auto-ionization for water is.
The reaction quotient Q has reached a maximum value and QK 2. Step 1 5 mL of the pink dilute solution is added into three test tubes. AA bB pP qQ where ABPQ are the chemicals involved and abpqare the coefficients in the balanced equation.
When a system is in chemical equilibrium the concentrations of the reactants and products are. The shift in equilibrium position of a chemical reaction with applied stress is determined. In chemical reaction chemical equilibrium refers to the phase when the concentration of both reactants and products are constant with no observable change with time.
The aim of the lab Chemical Equilibrium is to observe the effects of changes in concentrations of products and reactants on the position of the equilibrium of given chemical reactions. IntroductionGoals of Experiment In this lab we focus on the concept of equilibrium. Chemistry questions and answers.
When the conditions of a system at equilibrium are altered the system responds in such a way as to maintain the equilibrium. Equilibrium is something that all reactions reach at some point in time. This condition is expressed in the equilibrium constant Kc for the reaction.
But every chemical reaction has a point of equilibrium where the rate of the. According to this law the equilibrium condition is expressed by the equation. C formation of products equal in mass to the mass of the reactants D opposing reactions attaining equal rates.
Up to 24 cash back Investigating Equilibrium Name. When in equilibrium at a particular temperature a reaction mixture obeys the Law of Chemical Equilibrium which imposes a condition on the concentrations of reactants and products. Equilibrium equation for water is.
CHEM 1002 Laboratory 3. 21 February 2017 LAB SECTION. Formation of products equal in mass to the mass of the reactants.
Answer Correct Results NOTE. All questions in this color are observations you made in lab--you will receive full credit for these observations after your report has been submitted A. Pp 17-3 to 17-6.
Le Châteliers Principle NAME.

Solved Question 21 Chemical Equilibrium Is The Result Of Chegg Com
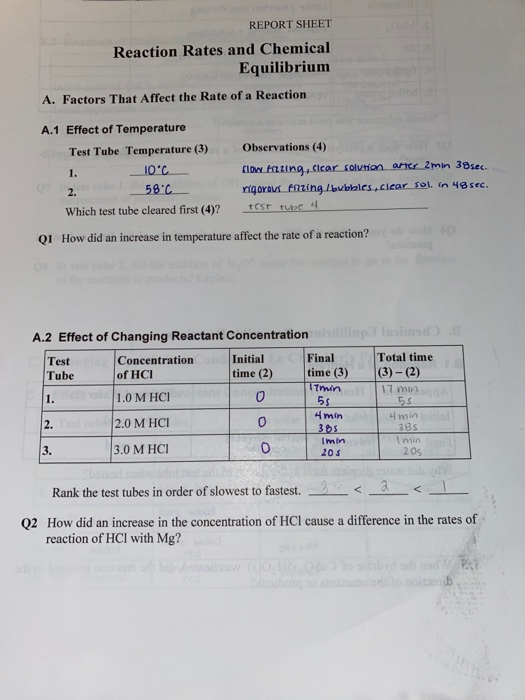
Solved Report Sheet Reaction Rates And Chemical Equilibrium Chegg Com
No comments for "Chemical Equilibrium Is the Result of Chegg"
Post a Comment